Since the outbreak of the new coronavirus (2019-nCoV), it has received extensive attention from the medical industry, and various manufacturers have actively invested in product research and development.
After sorting out, as of March 1, there have been more than 145 product information on the market that have successfully completed the development of new coronavirus (2019-nCoV) kits . This article selects 111 models with relatively complete information for analysis.
From the methodological point of view, the products can be divided into two categories: immunodiagnosis and nucleic acid detection:
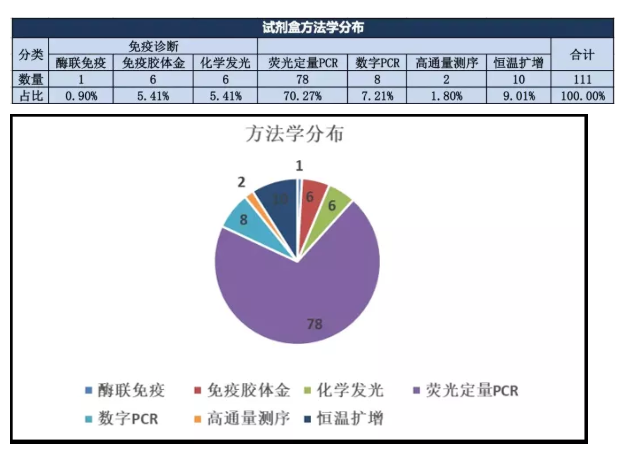
Among them, fluorescent quantitative PCR products have an advantage, mainly because the relevant R&D technology system is not complicated, the source of raw materials is relatively stable and abundant, and it is easier to quickly develop successful products. At the same time, it can be seen that a number of molecular technology enterprises are developing rapidly and the registration system is improving day by day.
Next, we will look at the advantages and disadvantages of the relevant methodologies:
1. Immunodiagnosis
Immunodiagnosis is a technology that applies the principle of specific antigen-antibody binding and uses specific markers for analysis and detection. Due to the existence of a window period (IgM, about 10 days with reference to SARS), it is difficult for this technique to detect positive results in the early stage of infection. The presence of interfering factors (heterophilic antibodies, rheumatoid factors, etc.) in the body, low immunity of patients, HOOK effect, etc. can also lead to false negative results. In addition, immunodiagnostic technology has good sensitivity but relatively poor specificity, and false-positive results may occur in the detection. Although its sensitivity and specificity are at a disadvantage compared to nucleic acid detection, due to the low requirements for laboratory equipment and personnel, this technology is suitable for large-scale detection at the grassroots level. The diagnosis is confirmed by nucleic acid testing.
1.1. Enzyme-linked immunosorbent assay (ELISA)
It has high sensitivity and specificity, and can detect both antibodies and soluble antigens. No special equipment is required, and the operation is simple and the detection cost is low, which is conducive to popularization. However, most of them are manual operations, the detection time is long, and the throughput is limited by the 96-well plate. In addition to the use of controls, blanks and quality controls, the actual number of patient samples tested is less than 96. At the same time, because the operating environment is mostly open, it is more likely to cause pollution. In the face of the new crown virus, this is a risk that needs to be paid attention to. According to the current situation, 4 brands have announced that they have successfully developed this product. For reasons of economy and other factors, this technology is very suitable for grass-roots testing institutions to carry out large-scale screening. It is expected that more manufacturers can develop and produce related products. .
1.2. Chemiluminescence (CLIA)
One of the most advanced immunoassay technologies at present, it has the advantages of high sensitivity, strong specificity, stable and rapid method, wide detection range, simple operation and high degree of automation; however, it often requires a brand-limited chemiluminescence instrument, and the cost of reagents is relatively high and cannot be opened. General, many small laboratories can not bear its cost pressure. At present, several representative luminescent reagent manufacturers have announced the development of 9 related products. With the corresponding chemiluminescence instrument, the detection speed can reach more than 200test/h, and there are even reports that the theoretical speed can reach 300test/h. It is expected to significantly improve the detection efficiency.
1.3. Immunocolloidal gold labeling technology (ICS)
It is simple in operation, quick in response (usually interpreting the results in a few minutes), and high in sensitivity. However, due to its poor sensitivity and inability to quantitatively detect and other weaknesses, its application scope is limited, and it is more suitable for bedside detection and rapid screening purposes. At present, more than 15 brands have announced that they have successfully developed related products, and all of them can detect the results within 15 minutes. If it can be applied to the clinic, it will play an important role in the auxiliary screening of the new coronavirus.

Location: Science and Technology Center, High-tech Zone, Shijiazhuang City, Hebei Province
Business Hotline: 186-3213-6937
Headquarters Tel: 0311-82970259
Email: sanshibio@126.com
